Ionic liquid-functionalized graphene oxide as an efficient support for the chiral salen Mn(iii) complex in asymmetric epoxidation of unfunctionalized olefins
Abstract
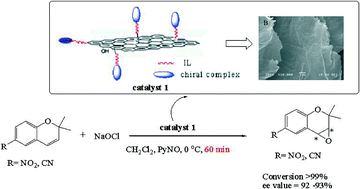
Imidazolium-based ionic liquid (IL)-functionalized graphene oxide (GO) was prepared and used to support chiral salen Mn(III) complexes. Technologies of characterization suggested that the intact chiral complex was covalently appended on flat planes and edge of the GO sheet through an imidazolium-based IL linker. The flexible layer-structure, as well as the active role of the IL linker in overall reaction, makes the novel heterogeneous catalyst efficient and universal for the enantioselective epoxidation of unfunctionalized olefins using NaClO as an oxidant. Remarkable enhancement of the reaction rate with excellent conversion (99%) was observed over a wide range of alkenes (styrene, α-methylstyrene, indene, 1,2-dihydronaphthalene, 6-cyano-2,2-dimethylchromene, and 6-nitro-2,2-dimethylchromene). The enantiomeric excess (ee) for epoxides was also encouraging (in the range of 73–93%), except for styrene (ee, 40%) and α-methylstyrene (ee, 44%). More importantly, the catalyst was perfectly stable and could be reused several times without significant loss of activity and enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...