The catalyzed hydrogen sorption mechanism in alkali alanates
Abstract
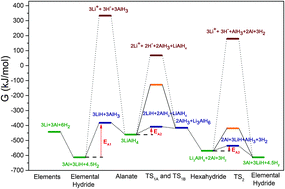
The hydrogen sorption pathways of alkali alanates were analyzed and a mechanism for the catalytic hydrogen sorption was developed. Gibbs free energy values of selected intermediate steps were calculated based on experimentally determined thermodynamic data (enthalpies and entropies) of individual hydrides: MAlH4, M3AlH6, and MH. The values of the activation energies, based on the intermediates M+, H−, MH, and AlH3, were obtained. The mechanism of the catalytic activity of Ti is finally clarified: we present an atomistic model, where MAlH4 desorbs hydrogen through the intermediates M+, H−, MH, and AlH3 to the hexahydride M3AlH6 and finally the elemental hydride MH. The catalyst acts as a bridge to transfer M+ and H− from MAlH4− to the neighboring AlH4−, forming AlH63− and finally isolated MH, leaving AlH3 behind, which spontaneously desorbs hydrogen to give Al and 1.5H2. The proposed mechanism is symmetric in the direction of hydrogen desorption as well as readsorption processes.

 Please wait while we load your content...
Please wait while we load your content...