Adsorbate enhancement of electron emission during the quenching of metastable CO at metal surfaces
Abstract
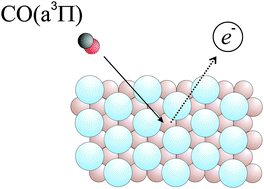
When electronically excited CO(a3Π) collides with a Au(111) surface, electron emission can be observed with a quantum efficiency of 0.13. We have studied the influence of Ar, Kr and Xe adsorption on the electron emission efficiency resulting from CO(a3Π) quenching. Surprisingly, a single monolayer (ML) of rare gas dramatically enhances electron emission. For Ar and Kr bilayers, emission efficiency is further enhanced and approaches unity. The quenching mechanism involves electron transfer from the metal to the CO(a3Π) molecule followed by electron emission from the molecule. The enhanced emission efficiency is due to the long range nature of the initial electron transfer process and the rare gas adlayer's ability to reflect the electron emitted by the transient CO anion. This work shows that CO(a3Π) quenching is a useful model system for investigating the fate of electronically excited molecules at surfaces.

 Please wait while we load your content...
Please wait while we load your content...