Insights into an autonomously formed oxygen-evacuated Cu2O electrode for the selective production of C2H4 from CO2†
Abstract
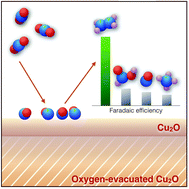
Electrochemical conversion of carbon dioxide (CO2) to small organic fuels (e.g. formate, methanol, ethylene, ethanol) is touted as one of the most promising approaches for solving the problems of climate change and energy security. In this study, we report the highly efficient electrochemical reduction of CO2 using cuprous oxide (Cu2O) electrodes to produce ethylene (C2H4) primarily. During CO2 electrolysis using electrodeposited Cu2O on a carbon electrode, we observe the transformation of a compact metal oxide layer to a metal oxide structure with oxygen vacant sites at the bulk region. In contrast to previous studies, our results clearly indicate that Cu2O remains at the surface of the catalyst and it efficiently catalyzes the conversion process of CO2 at low overpotential, exhibiting a high selective faradaic efficiency of over 20% towards C2H4 formation even in long-term electrolysis.

 Please wait while we load your content...
Please wait while we load your content...