A furosemide–isonicotinamide cocrystal: an investigation of properties and extensive structural disorder†
Abstract
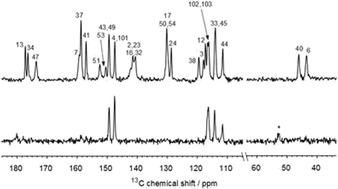
Furosemide is a loop diuretic drug marketed in solid form which suffers from low solubility and low permeability. The pharmaceutically relevant properties of a recently described furosemide–isonicotinamide 2 : 1 cocrystal (2FS–INA) were investigated and compared with those of other known furosemide cocrystals. The intrinsic dissolution rate of 2FS–INA was found to be very similar to that of commercial FS, while its equilibrium solubility was 5.6 times higher than that of pure FS. The extensive structural disorder in 2FS–INA observed by diffraction methods was also investigated by variable-temperature solid-state NMR in conjunction with first principles calculations. 15N NMR confirmed the absence of proton disorder in the short OH⋯N hydrogen bond. The disordered sulphonamide group was found to be dynamic by variable temperature 2H experiments, involving fast exchange of the sulphonamide NH2 protons combined with a rotation of the whole sulphonamide group about the C–S bond. The disorder of the furan rings of both the unique furosemide molecules was also found to be dynamic by 13C experiments, with approximately the same activation barrier for both rings.

 Please wait while we load your content...
Please wait while we load your content...