Effective 1,5-stereocontrol in Pd(0)/InI promoted reactions of chiral N-Ts-4-vinylazetidin-2-ones with aldehydes. An efficient entry into nonracemic semi-protected (3Z)-2,6-anti-enediols†
Abstract
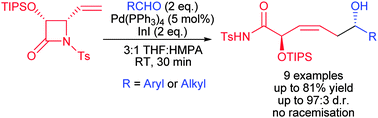
ε-Amido-allylindiums generated in situ from N-Ts-4-vinylazetidin-2-ones in the presence of 2 eq. of InI and catalytic amounts of Pd(PPh3)4 react with a number of aromatic and aliphatic aldehydes with effective remote 1,5-stereocontrol to afford (3Z)-2,6-anti-enediols as major products in good yields and with excellent diastereoselectivity.

 Please wait while we load your content...
Please wait while we load your content...