The catalytic asymmetric synthesis of tetrahydropyridazines via inverse electron-demand aza-Diels–Alder reaction of enol ethers with azoalkenes†
Abstract
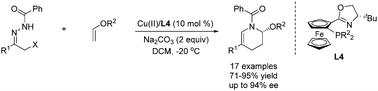
A highly efficient Cu(II)-catalyzed enantioselective inverse-electron-demand aza-Diels–Alder reaction of in situ formed azoalkenes with enol ethers is reported. This methodology provides a facile entry to biologically important and enantioenriched tetrahydropyridazine derivatives in generally good yield (up to 95% yield) with good to excellent enantioselectivity (up to 94% ee).

 Please wait while we load your content...
Please wait while we load your content...