Synthetic heparan sulfate dodecasaccharides reveal single sulfation site interconverts CXCL8 and CXCL12 chemokine biology†
Abstract
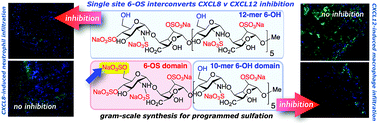
The multigram-scale synthesis of a sulfation-site programmed heparin-like dodecasaccharide is described. Evaluation alongside dodecasaccharides lacking this single glucosamine O6-sulfation, or having per-O6-sulfation, shows that site-specific modification of the terminal glucosamine dramatically interconverts regulation of in vitro and in vivo biology mediated by the two important chemokines, CXCL12 (SDF1α) or CXCL8 (IL-8).


 Please wait while we load your content...
Please wait while we load your content...