Insight into the esterase like activity demonstrated by an imidazole appended self-assembling hydrogelator†
Abstract
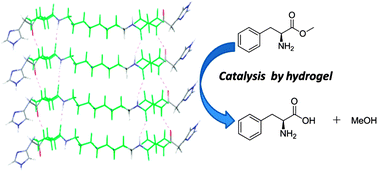
A low molecular weight hydrogelator with a covalently appended imidazole moiety is reported. Capable of percolating water in the pH range of 6 to 8, it proves to be an efficient catalyst upon self-assembly, showing Michaelis–Menten type kinetics. Activities at different pH values correlated with dramatic structural changes were observed. It can hydrolyse p-nitrophenyl acetate (pNPA) as well as inactivated esters, and L and D-phenylalanine methyl esters. The enhanced activity can be related to the conglomeration of catalytic groups upon aggregation resulting in their close proximity and the formation of hydrophobic pockets.

 Please wait while we load your content...
Please wait while we load your content...