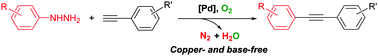
Palladium-catalyzed aerobic oxidative cross-coupling of arylhydrazines with terminal alkynes†
Abstract
The palladium-catalyzed Sonogashira-type aerobic oxidative coupling of arylhydrazines with terminal alkynes via C–N bond cleavage has been developed; internal alkynes were afforded with a broad substrate scope. This reaction proceeds under copper- and base-free conditions with molecular oxygen as the sole oxidant and nitrogen and water as the only by-products.

 Please wait while we load your content...
Please wait while we load your content...