Catalytic, oxidant-free, direct olefination of alcohols using Wittig reagents†
Abstract
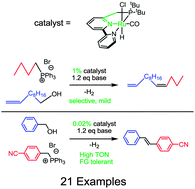
Reported here is the catalytic, acceptorless coupling of alcohols with in situ generated, non-stabilized phosphonium ylides to form olefins as major products. The reaction uses low catalyst loadings and does not require added oxidants. Hydrogenation of the product is minimized and the reaction leads to Z (aliphatic) or E (benzylic) stereospecificity.

 Please wait while we load your content...
Please wait while we load your content...