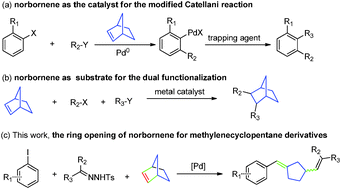
Palladium-catalyzed ring opening of norbornene: efficient synthesis of methylenecyclopentane derivatives†
Abstract
The first palladium-catalyzed ring opening of norbornene to prepare methylenecyclopentane derivatives has been established. The process, which uses readily available aryl iodides, tosylhydrazones and norbornene as starting materials, likely takes place via tandem Heck-type coupling, palladium carbene migratory insertion, C–C bond cleavage and the β-hydride elimination pathway in a single synthetic sequence.

 Please wait while we load your content...
Please wait while we load your content...