Asymmetric palladium-catalyzed umpolung cyclization of allylic acetate-aldehyde using formate as a reductant†
Abstract
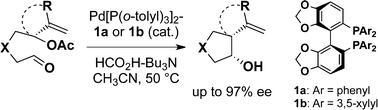
Palladium/chiral diphosphine-catalyzed umpolung cyclization of allylic acetate-aldehyde using formate as a terminal reductant affords cis-disubstituted pyrrolidine, tetrahydrofuran, and spiro carbocycle in high enantioselectivity. The formate does not cause allylpalladium reduction under the catalysis. The highly stereoselective cyclization would proceed through a cationic η1-allylpalladium ligated by diphosphine.

 Please wait while we load your content...
Please wait while we load your content...