Nickel-catalyzed and benzoic acid-promoted direct sulfenylation of unactivated arenes†
Abstract
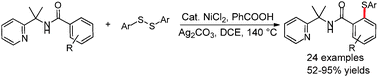
A nickel-catalyzed and benzoic acid-promoted direct sulfenylation of unactivated arenes using removable 2-(pyridine-2-yl)-isopropylamine as a directing group is described. This strategy provides an efficient access to valuable aryl sulfides with ample substrate scope and a high degree of functional group tolerance.

 Please wait while we load your content...
Please wait while we load your content...