Salicylic acids as readily available starting materials for the synthesis of meta-substituted biaryls†
Abstract
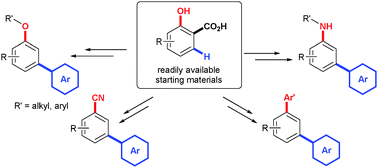
Salicylic acids are shown to be readily available and versatile starting materials that easily undergo a tandem arylation–protodecarboxylation process under Pd-catalysis. The corresponding meta-arylphenols can subsequently be easily transformed into a variety of meta-functionalized biaryls, highlighting the versatility of this approach to access this structural motif.

 Please wait while we load your content...
Please wait while we load your content...