Aptamer based dispersion assay using tunable resistive pulse sensing (TRPS)†
Abstract
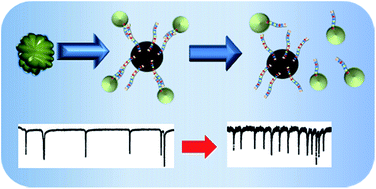
Aggregates of micron sized beads were formed by the binding of anti-thrombin aptamer to its complement. The addition of the thrombin protein target caused a concentration-dependant dispersion of these aggregates, and their number was measured by tunable resistive pulse sensing. The technique allowed the detection of thrombin down to sub picomolar concentrations, and an increase in sensitivity over previous assays on the same platform. The sensitivity of the assay is attributed to each thrombin protein disrupting multiple aggregates resulting in a signal amplification.


 Please wait while we load your content...
Please wait while we load your content...