Incorporation of tin affects crystallization, morphology, and crystal composition of Sn-Beta†
Abstract
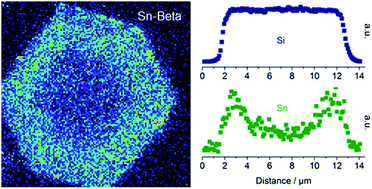
The crystallization of Sn-Beta in fluoride medium is greatly influenced by the amount and type of tin source present in the synthesis gel. By varying the amount of tin in the form of tin(IV) chloride pentahydrate, the time required for crystallization was studied. It was found that tin not only drastically affects the time required for crystallization, but also that the presence of tin changes the morphology of the formed Sn-Beta crystals. For low amounts of tin (Si/Sn = 400) crystallization occurs within four days and the Sn-Beta crystals are capped bipyramidal in shape, whereas for high amounts of tin (Si/Sn = 100) it takes about sixty days to reach full crystallinity and the resulting crystals are highly truncated, almost plate-like in shape. Using SEM-WDS to investigate the tin distribution along transverse sections of the Sn-Beta crystals, a gradient distribution of tin was found in all cases. It was observed that the tin density in the outer parts of the Sn-Beta crystals is roughly twice as high as in the tin depleted core of the crystals. Sn-Beta was found to obtain its maximum catalytic activity for the conversion of dihydroxyacetone to methyl lactate close to the minimum time required for obtaining full crystallinity. At excessive crystallization times, the catalytic activity decreased, presumably due to Ostwald ripening.

 Please wait while we load your content...
Please wait while we load your content...