P-type NaxNi0.22Co0.11Mn0.66O2 materials: linking synthesis with structure and electrochemical performance†
Abstract
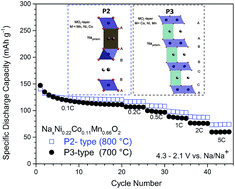
P-type layered oxides are promising cathode materials for sodium-ion batteries and a wide variety of compounds have been investigated so far. Nevertheless, detailed studies on how to link synthesis temperature, structure and electrochemistry are still rare. Herein, we present a study on P-type NaxNi0.22Co0.11Mn0.66O2 materials, investigating the influence of synthesis temperature on their structure and electrochemical performance. The change of annealing temperature leads to various materials of different morphologies and either P3-type (700 °C), P3/P2-type (750 °C) or P2-type (800–900 °C) structure. Galvanostatic cycling of P3-type materials revealed high initial capacities but also a high capacity fade per cycle leading to a poor long-term cycling performance. In contrast, pure P2-type NaxNi0.22Co0.11Mn0.66O2, synthesized at 800 °C, exhibits lower initial capacities but a stable cycling performance, underlined by a good rate capability, high coulombic efficiencies and high average discharge capacity (117 mA h g−1) and discharge voltage (3.30 V vs. Na/Na+) for 200 cycles.
- This article is part of the themed collection: 2014 Journal of Materials Chemistry A Hot Articles

 Please wait while we load your content...
Please wait while we load your content...