Systematic electrochemical oxidative doping of P3HT to probe interfacial charge transfer across polymer–fullerene interfaces†
Abstract
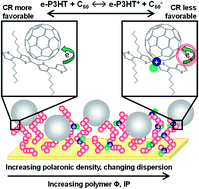
This work demonstrates the detection and control of interfacial charge transfer across polymer–fullerene interfaces relevant to organic electronic platforms, including solar cells and photodetectors. Electrochemical deposition of poly(3-hexylthiophene) (e-P3HT) and subsequent electrochemical oxidation to systematically vary the fraction of oxidized thiophene (e-P3HT+) was used to form donor polymer films. The fullerene electron acceptor C60 was vacuum deposited onto the e-P3HT, and interfacial interactions were monitored with optical and photoelectron spectroscopy. Charge redistribution (sub-stoichiometric or even stoichiometric electron transfer) from e-P3HT to C60 was observed when the initial fraction of e-P3HT+ was low, as evidenced by the formation of new polaronic species and simultaneous n-doping of the C60. These charge transfer results are expected to impact interfacial rates of free carrier generation and recombination, as well as competing charge transport processes, particularly in thin film devices (<60 nm). While charge transfer of this sort has been previously observed, the ability to control the extent of charge redistribution through the systematic oxidation of the thiophene species demonstrates additional chemical tunability that can further increase the functionality of polymer–fullerene type II heterojunctions. Collectively, this work highlights the need to characterize and strategically manipulate nanoscale deviations from bulk properties in the future rational design of functional organic electronics.

 Please wait while we load your content...
Please wait while we load your content...