The role of electronic interaction in the use of Ag and Mn3O4 hybrid nanocrystals covalently coupled with carbon as advanced oxygen reduction electrocatalysts†
Abstract
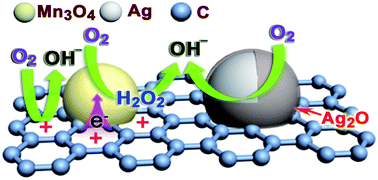
Achieving synergy between inexpensive metals and metal oxides is a key challenge for the development of highly active, economical composites as next-generation catalysts for oxygen reduction reaction (ORR). We have synthesized highly dispersed Ag and Mn3O4 nanocrystals covalently coupled with carbon black via a simple thermal decomposition of AgNO3 and Mn(NO3)2 precursors at elevated temperatures. The resulting Ag and Mn3O4 nanocrystals are located separately on the carbon, but in close proximity to each other. Electrocatalysis experiments in an alkaline solution reveal a remarkably improved electrocatalytic activity and prolonged long-term durability for the Ag–Mn3O4/C composite relative to the Ag/C (90 wt%). Moreover, X-ray photoelectron spectroscopy (XPS) and X-ray absorption spectroscopy (XAS) demonstrate that the unique electronic structures of the composite could be tuned by the substantial electron transfer between the two nanoparticles through the common carbon support, which highly correlates with the enhanced ORR performance. The exact origin of the improved ORR activity may be associated with the favorable formation of monolayer Ag2O film and promoted active oxygen adsorption on the Ag surfaces as a result of the particle-to-particle ligand and ensemble effects between Ag and Mn3O4 phases in this composite.

 Please wait while we load your content...
Please wait while we load your content...