Tuning “thiol-ene” reactions toward controlled symmetry breaking in polyhedral oligomeric silsesquioxanes†
Abstract
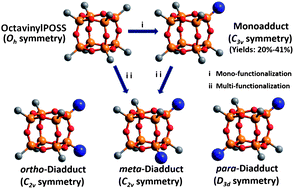
The convenient synthesis of nano-building blocks with strategically placed functional groups constitutes a fundamental challenge in nano-science. Here, we describe the facile preparation of a library of mono- and di-functional (containing three isomers) polyhedral oligomeric silsesquioxane (POSS) building blocks with different symmetries (C3v, C2v, and D3d) using thiol-ene chemistry. The method is straightforward and general, possessing many advantages including minimum set-up, simple work-up, and a short reaction time (about 0.5 h). It facilitates the precise introduction of a large variety of functional groups to desired sites of the POSS cage. The yields of the monoadducts increase significantly using stoichiometric amounts of bulky ligands. Regio-selective di-functionalization of the POSS cage was also attempted using bulky thiol ligands, such as a thiol-functionalized POSS. Electrospray ionization (ESI) mass spectrometry coupled with travelling wave ion mobility (TWIM) separation revealed that the majority of diadducts are para-compounds (∼59%), although meta-compounds (∼20%) and ortho-compounds (∼21%) are also present. Therefore, the thiol-ene reaction provides a robust approach for the convenient synthesis of mono-functional POSS derivatives and, potentially, of regio-selective multi-functionalized POSS derivatives as versatile nano-building blocks.

 Please wait while we load your content...
Please wait while we load your content...