A combined experimental and computational study of supramolecular assemblies in ternary copper(ii) complexes with a tetradentate N4 donor Schiff base and halides†
Abstract
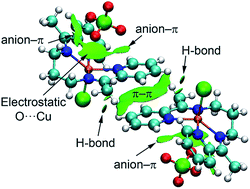
Three new copper(II) complexes, [Cu(L)(Cl)]ClO4 (1), [Cu(L)(Br)]ClO4 (2) and [Cu(L)(I)]ClO4 (3), have been prepared from a tetradentate symmetrical Schiff base, N,N′-bis-(1-pyridin-2-yl-ethylidene)-propane-1,3-diamine (L), and characterized by elemental analysis, IR and UV-Vis spectroscopy and single-crystal X-ray diffraction studies. Extended supra-molecular networks were generated through different weak non-covalent forces. Density functional theory (DFT) calculations were employed to estimate the contribution of each interaction in the formation of the assembly using several theoretical models. The interplay between the anion–π and π–π interactions is also analyzed and a mutual reinforcement of both interactions is demonstrated. The assignment of the contribution of each interaction and its mutual influence is certainly important to shed light on the delicate mechanism that governs the molecular recognition and crystal packing.


 Please wait while we load your content...
Please wait while we load your content...