Source and major species of CHx (x = 1–3) in acetic acid synthesis from methane–syngas on Rh catalyst: a theoretical study†
Abstract
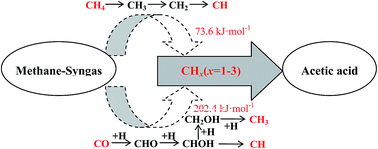
Density functional calculations have been carried out to investigate the source and major species of CHx (x = 1–3) involved in acetic acid synthesis from methane–syngas on the Rh(111) surface. All possible formation pathways of CHx (x = 1–3) from methane and syngas have been systematically investigated. For CHx formation from methane, our results show that CH is the most abundant species; for CHx formation from syngas, all CHx (x = 1–3) species form from CHO by CO hydrogenation, and the optimal formation routes of CHx show that CH and CH3 are the most abundant species rather than CH2 and CH3OH. On the other hand, CH formed by methane is more favourable than CH and CH3 formed by syngas; meanwhile, CO insertion into CHx species to form C2 oxygenates as acetic acid precursors is more favourable than CO hydrogenation to CH and CH3. As a result, in acetic acid synthesis from methane–syngas, CHx (x = 1–3) species come from methane rather than syngas, and the corresponding primary species is CH. In addition, the CO in syngas is predominantly responsible for insertion reactions that produce CHCO, which is a C2 oxygenate precursor leading to the formation of acetic acid. Furthermore, microkinetic modelling analysis shows that the major product of acetic acid synthesis from methane–syngas on the Rh(111) surface is CH3COOH, and that the production of CH3OH cannot compete with that of CH3COOH.

 Please wait while we load your content...
Please wait while we load your content...