Synthesis and characterization of robust three-dimensional chiral metal sulfates†
Abstract
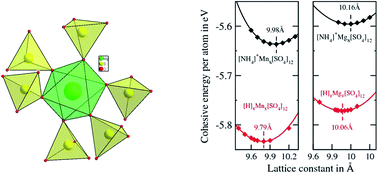
Two chiral three-dimensional metal sulfates of the compositions [NH4]8[Mn8(SO4)12], (1) and [NH4]8[Mg8(SO4)12], (2) of the langbeinites family have been synthesized under hydro/solvothermal conditions and studied using first principles within the framework of density functional theory (DFT). Both the compounds 1 and 2 are isostructural and crystallize in the cubic chiral space group P2(1)3. In the presence of NH4+ cations, the frameworks of 1 and 2 have three-dimensional structures formed by the corner sharing of metal octahedra and sulfate tetrahedra through M–O–S linkages, which leads to a pinwheel arrangement of the metal octahedra surrounded by six sulfate tetrahedra. Both 1 and 2 are observed to retain their structures upon the thermal decomposition of ammonium ion, resulting into the compositions [H]8[Mn8(SO4)12] and [H]8[Mg8(SO4)12], respectively. The magnetic measurement of 1 shows a paramagnetic behaviour and the hydrogen adsorption of 1 and 2 shows 0.45 and 0.7 wt%, respectively at 77 K. The proton conductivity of 1 under various relative humidities (RH) shows 2.6 × 10−7 S cm−1 at RH = 80% and reaches to 3.1 × 10−4 S cm−1 at RH = 90%. First-principles calculations suggest structural evolution along with the retention of framework structures of similar stabilities by both the compounds after de-ammoniation.

 Please wait while we load your content...
Please wait while we load your content...