Rational synthesis of bis(hexyloxy)-tetra(hydroxy)-triphenylenes and their derivatives†
Abstract
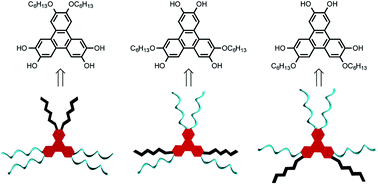
A straightforward, reliable, and scalable synthesis of rationally designed, mixed-substituent triphenylene derivatives from ortho-terphenyl precursors is described. Three isomers of bis(hexyloxy)-tetrahydroxy triphenylenes were synthesized and functionalized with monomethyl di(ethylene glycol) chains to provide new amphiphilic, mixed substituent triphenylenes. Oxidative triphenylene annulation, tetra-ol formation, and subsequent functionalization were supported by significant changes in phase and melting point, and confirmed by mass spectrometry, differential scanning calorimetry, and UV/Vis, 1H, and 13C NMR spectroscopies. The thermal phase properties of amphiphilic mixed-substituent triphenylene derivatives were found to vary between the different isomers, demonstrating how small changes in substitution pattern can result in significant differences in mesogenic behavior. The controlled synthetic route to de novo designed triphenylene derivatives is dependable, wide in scope, and can be applied to the synthesis of a vast array of other mixed-substituent triphenylene derivatives, thus enabling the preparation of libraries of novel triphenylene and triphenylene-containing materials.

 Please wait while we load your content...
Please wait while we load your content...