Relaxation dynamics in lens crystallin proteins: a dielectric and thermodynamic approach using TDR†
Abstract
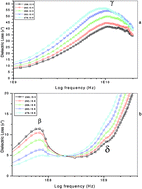
Dielectric relaxation of water in biological systems, such as proteins and DNA, can often be described by two different time constants, one in the picosecond and the other in the nanosecond regime. In the present work, we report the temperature dependence of the relaxation dynamics, dielectric permittivity (ε′) and loss (ε′′) of aqueous lens proteins (crystalline) in the goat eye lens. The measurements have been carried out in the frequency range from 10 MHz to 20 GHz using TDR and in the temperature region from 298.15 K to 278.15 K. We analyse the three dispersion regions commonly found in protein solutions, usually termed β-, γ- and δ-relaxation. The β-relaxation, occurring in the frequency range between 70 and 100 MHz, and the γ-relaxation between 15 and 18 GHz can be attributed to the rotation of the polar protein molecules in their aqueous medium and the reorientational motion of the free water molecules respectively. The nature of δ-relaxation, which is often ascribed to the motion of bound water molecules, is not yet fully understood. Here, we provide data on the temperature dependence of dielectric and thermodynamic parameters of all three detected processes, β-, γ- and δ-relaxation. We found significant temperature dependence of the dielectric and thermodynamic parameters of the aqueous proteins, indicating conformational changes.

 Please wait while we load your content...
Please wait while we load your content...