Pyrolysis behavior of biomass with different Ca-based additives
Abstract
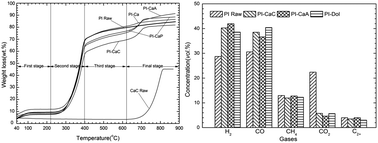
CaO is an important catalyst in biomass gasification and pyrolysis. However, CaO can stem from many precursors. And, the effect between different precursors has still not been studied. Calcined natural limestone, natural dolomite, calcium oxide, calcium carbonate, calcium acetate, and calcium propionate were used to investigate their pyrolysis reactivity of biomass (peanut shells and pine sawdust) at high temperature (>800 °C). Experiments were conducted using a thermogravimetric apparatus and a fixed-bed pyrolysis system. Pine sawdust displayed higher pyrolysis reactivity than peanut shells. During pyrolysis, Ca-based additives fixed carbon dioxide mainly in the temperature range of 300–700 °C. By addition of Ca-based additives, the concentrations of hydrogen and carbon monoxide were increased and the concentrations of methane and carbon dioxide (over 18 vol%) were decreased. De-carbonation started around 700 °C. The emission of CO2 by de-carbonation promoted the reduction of char by the Boudouard reaction and methane by the dry reforming reaction. The total pyrolysis conversion decreased as PI-Dol > PI-CaA > PI-Lime > PI-Ca > PI-CaP > PI Raw > PI-CaC. The excellent pyrolysis reactivity of biomass in the presence of calcined calcium acetate can be ascribed to its preeminent physical structure. Addition of Ca-based additives increased the activation energy of the main devolatilization region. The activation energy of biomass with calcined natural dolomite, about 110 kJ mol−1, was much lower than that of other Ca-based additives studied at high temperatures. Pyrolysis of the pure biomass sample can be simply hypothesized as a first order reaction, but it varied significantly with the addition of additives.

 Please wait while we load your content...
Please wait while we load your content...