Use of electrochemical measurements to investigate the porosity of ultra-thin Al2O3 films prepared by atomic layer deposition
Abstract
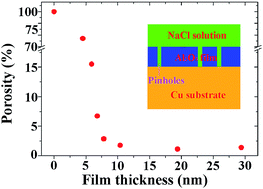
The porosity of ultra-thin alumina (Al2O3) films was determined by electrochemical measurements. The Al2O3 films were prepared by atomic layer deposition (ALD) on a copper substrate using trimethyl aluminum and water as the precursors. The copper substrate was fine-polished to decrease the influence of any surface defects on the porosity. 30 to 300 ALD cycles were performed to obtain films with a thickness in the range 4.5–29.4 nm. Auger electron spectroscopy results revealed that the Al2O3 films prepared by ALD were stoichiometric and showed low substrate sensitivity. The results obtained by potentiodynamic polarization showed that the porosity of the Al2O3 films decreased with film thickness. However, when the film thickness increased to 7.8 nm, the film porosity became stable. With further increases in the film thickness, the porosity decreased slowly. The copper substrate was well protected as a result of the low porosity of the 7.8 nm Al2O3 film. No obvious corrosion was observed in the scanning electron microscopy images.

 Please wait while we load your content...
Please wait while we load your content...