Electrochemical synthesis of poly(3,4-ethylenedioxythiophene) in aqueous dispersion of high porosity reduced graphene oxide†
Abstract
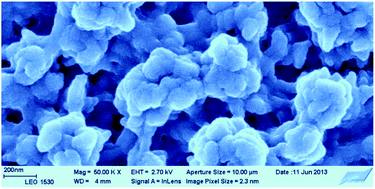
We report here the one-step electrochemical synthesis of poly(3,4-ethylenedioxythiophene) (PEDOT) in an aqueous dispersion of reduced graphene oxide (rGO). The electrochemical polymerization is carried out at 1.05 V in aqueous media in the presence of 10−2 M 3,4-ethylenedioxythiophene and 1 g L−1 rGO. Unlike composites of PEDOT and graphene oxide or poly(styrene sulfonate) which have rather smooth and non-porous surface morphologies, the scanning electron microscopy images reveal that the PEDOT composite films obtained in this work have uniformly porous and open surface morphology. X-ray photoelectron spectroscopy (XPS) showed that rGO had initially a low concentration of oxygen-containing surface groups (C : O ratio = 6.5), but both FTIR spectroscopy and XPS showed that the electropolymerization resulted in the formation of OH groups in the composite film. Characterization with cyclic voltammetry and electrochemical impedance spectroscopy demonstrates that the composite films behave almost like ideal capacitors having an areal capacitance of 12.2 mF cm−2. The composite films had a very good potential cycling stability in 0.1 M KCl with only 12.4% degradation of the capacitance in a three-electrode cell after 3000 cycles between −0.5 and 0.5 V. The degradation was higher (32.8%) in the broader potential range of −0.8 and 0.7 V.

 Please wait while we load your content...
Please wait while we load your content...