Spectral properties of 4-(4-hydroxy-1-naphthylazo)benzenesulfonic acid and its application for colorimetric determination of trace Fe3+
Abstract
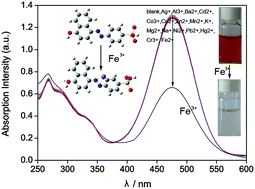
A multifunctional dye, 4-(4-hydroxy-1-naphthylazo)benzenesulfonic acid (HNABA) was identified and synthesized. The dye, combining hydroxyl, azo and carboxyl groups, possessed excellent optical absorption properties changing with pH, solvent and coexisting metal ions. In particular, its spectral properties remained extremely stable under acid or neutral conditions and it was effectively applied for colorimetric determination of Fe3+ from brick-red to light red in aqueous solution under physiological pH conditions (pH 7.0). Under the optimum conditions, the detection possessed a linear range of 9.5–400 × 10−8 mol L−1 with a correlation coefficient (R) of 0.9938 and a limit detection (3σ, n = 20) of 4.2 × 10−9 mol L−1. The relative standard deviation (R.S.D.) was lower than 3.5% (n = 5). The proposed method was successfully used to determine trace Fe3+ in three real environmental water samples. The mechanism of action between HNABA and Fe3+ ion is discussed in detail.

 Please wait while we load your content...
Please wait while we load your content...