Zinc iodide: a mild and efficient catalyst for one-pot synthesis of aminoindolizines via sequential A3 coupling/cycloisomerization†
Abstract
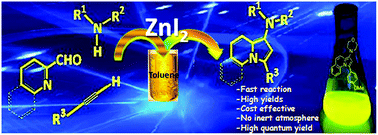
ZnI2 was found to be an efficient catalyst for the synthesis of indolizine derivatives by a three-component coupling of pyridine-2-carboxaldehyde/quinoline-2-carboxaldehyde, secondary amines, and terminal alkynes in high yields. This protocol is compatible with a wide range of substrates and is expected to find broad applications due to its operational simplicity, shorter reaction time and low cost. A preliminary photophysical study showed that synthesized morpholinopyrrolo[1,2-a]quinolines represent a new class of fluorophore with high quantum yield.

 Please wait while we load your content...
Please wait while we load your content...