Imino exchange reaction in a dearomatization strategy: synthesis of N-acyl diarylamines and phenothiazines from two anilines†
Abstract
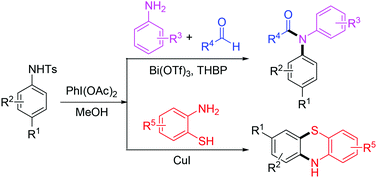
N-Sulfonyl cyclohexadienimines generated from an iodine(III)-induced oxidative dearomatization of N-sulfonyl protected para-substituted anilines are ready to undergo an imino exchange reaction with another aniline, which provides an alternative way to access N-acyl diarylamines and phenothiazines.

 Please wait while we load your content...
Please wait while we load your content...