Highly stereoselective construction of adjacent tetrasubstituted carbon stereogenic centres via an organocatalytic Mukaiyama-aldol reaction of monofluorinated silyl enol ethers to isatins†
Abstract
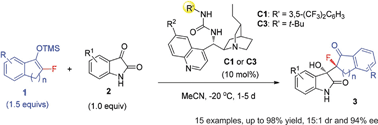
We report the first organocatalytic activation of monofluorinated silyl enol ethers 1, by nucleophilic tertiary amine catalysis, to develop asymmetric reactions for the construction of fully substituted chiral carbons featuring a C–F bond. Accordingly, a highly stereoselective Mukaiyama-aldol reaction of isatins to furnish hydroxyoxindoles bearing two adjacent tetrasubstituted carbon stereocenters is developed.

 Please wait while we load your content...
Please wait while we load your content...