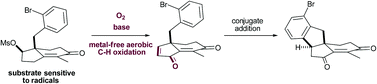
Abstract
A metal-free procedure is described for the aerobic and complete C–H methylene oxidation of Hajos–Parrish enones to versatile dihydroindenediones. The synthetic utility of these substrates was illustrated by converting them into highly substituted indanes after an intramolecular Friedel–Crafts conjugate addition. Importantly, the aerobic oxidation was compatible with substrates sensitive to radicals.
- This article is part of the themed collection: In Celebration of Max Malacria’s 65th Birthday

 Please wait while we load your content...
Please wait while we load your content...