Fluorescence from bisaryl-substituted maleimide derivatives†
Abstract
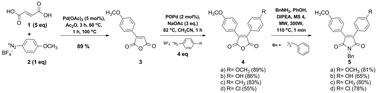
A series of bisaryl-substituted fluorescent maleimides was synthesized via the Heck arylation. The compounds showed broad fluorescence emission bands in the visible region, a large Stokes shift in polar solvents and emission quantum yields varying from 0.04 to 0.71, depending on the structure and solvent medium. The difference in dipole moments of ground and excited electronic states of about 12 Debye is ascribed to a substantial charge shift and push–pull character of bisaryl-substituted maleimides. The fluorescence decays of N-benzyl-3,4-bis(4-methoxyphenyl)-1H-pyrrole-2,5-dione (compound 5a) are biexponential with short (1.3–7.6 ns) and long lived (11.5–13.6 ns) components in polar solvents, but in 1,4-dioxane and THF the decays become single exponential. On the other hand, N-benzyl-3-(4-methoxyphenyl)-4-(4-hydroxyphenyl)-1H-pyrrole-2,5-dione (compound 5b) exhibited a biexponential decay in DMSO and in DMF with much shorter decay components, and such behavior indicated a charge shift process combined with solvent assisted proton transfer in the excited state.
- This article is part of the themed collection: International Conference on Photochemistry 2013 (ICP2013) keynote lectures

 Please wait while we load your content...
Please wait while we load your content...