Orthogonal dual-click diyne for CuAAC and/or SPAAC couplings†
Abstract
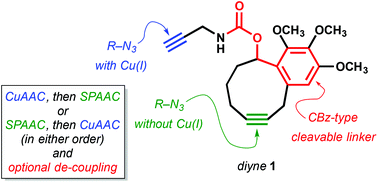
Carbamate-tethered propargyl and benzocyclononyne moieties within a single molecular unit undergo cycloaddition with azides under complementary CuAAC and/or SPAAC coupling conditions. The carbamate linker can be cleaved by analogy to the –CBz protecting group for click capture-and-release applications.

 Please wait while we load your content...
Please wait while we load your content...