Abstract
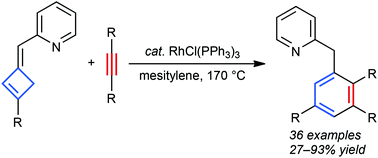
Cyclobutenes with 2-pyridylmethylene groups at the 3 position underwent an intermolecular alkyne insertion reaction in the presence of a rhodium(I) catalyst at 170 °C to afford substituted benzenes. Among the different 2-pyridylmethylene groups examined, 3-methyl-2-pyridyl derivatives showed superior activity and readily coupled with various alkynes, including sterically demanding, heteroaromatic and terminal alkynes.


 Please wait while we load your content...
Please wait while we load your content...