Copper-catalyzed nucleophilic trifluoromethylation of benzylic chlorides†
Abstract
Reactions of primary and secondary benzylic chlorides with trifluoromethyltrimethylsilane in the presence of a catalytic amount of copper(I) thiophene-2-carboxylate (CuTC) have been found to give the corresponding benzylic trifluoromethylated products in good to high yields.
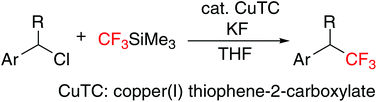

 Please wait while we load your content...
Please wait while we load your content...