Palladium-catalyzed oxidative carbonylative coupling of arylboronic acids with terminal alkynes to alkynones†
Abstract
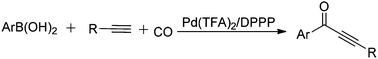
We describe here an interesting palladium-catalyzed oxidative carbonylation of arylboronic acids with terminal alkynes. By the appropriate combination of a palladium salt, a ligand, and an oxidant, the desired alkynones were isolated in moderate to good yields. Notably, all the reactions were performed at room temperature, and moisture and air can be tolerated by this procedure. More importantly, this is the first example of oxidative carbonylative coupling of arylboronic acids with alkynes which filled the missing link in carbonylative coupling reactions.

 Please wait while we load your content...
Please wait while we load your content...