Electrophilic sulfhydration of 8-nitro-cGMP involves sulfane sulfur†
Abstract
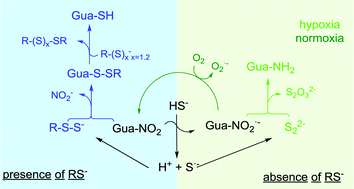
The formation of 8-SH-cGMP from the reaction between hydrogen sulfide and 8-nitro-guanosine-3′,5′-cyclic monophosphate in the presence of thiols does not take place by nucleophilic attack of the hydrosulfide anion, as previously proposed, but first involves the formation of reactive species containing sulfane sulfur, like persulfides.

 Please wait while we load your content...
Please wait while we load your content...