An experimental and theoretical study of reaction mechanisms between nitriles and hydroxylamine†
Abstract
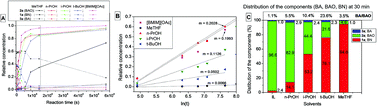
The industrially relevant reaction between nitriles and hydroxylamine yielding amidoximes was studied in different molecular solvents and in ionic liquids. In industry, this procedure is carried out on the ton scale in alcohol solutions and the above transformation produces a significant amount of unexpected amide by-product, depending on the nature of the nitrile, which can cause further analytical and purification issues. Although there were earlier attempts to propose mechanisms for this transformation, the real reaction pathway is still under discussion. A new detailed reaction mechanistic explanation, based on theoretical and experimental proof, is given to augment the former mechanisms, which allowed us to find a more efficient, side-product free procedure. Interpreting the theoretical results obtained, it was shown that the application of specific imidazolium, phosphonium and quaternary ammonium based ionic liquids could decrease simultaneously the reaction time while eliminating the amide side-product, leading to the targeted product selectively. This robust and economic procedure now affords a fast, selective amide free synthesis of amidoximes.

 Please wait while we load your content...
Please wait while we load your content...