Conformational effects due to stereochemistry and C3-substituents in xylopyranoside derivatives as studied by NMR spectroscopy
Abstract
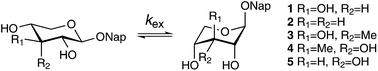
Glycosaminoglycans contain a β-D-xylopyranose residue at its reducing end, which links the polysaccharide to the protein in proteoglycans. 2-Naphthyl β-D-xylopyranosides have shown inhibition of tumor growth and we herein investigate conformation and dynamics of compounds structurally and stereochemically modified at the C3 position as well as the influence of solvent. The 3-deoxygenated compound, the 3-C-methyl-substituted β-D-xylopyranoside, β-D-ribopyranoside, the 3-C-methyl-substituted β-D-ribopyranoside as well as 2-naphthyl β-D-xylopyranoside were analyzed by NMR spectroscopy. Conformational equilibria were dependent on the solvent of choice, either methanol-d4 or chloroform-d, with mainly 4C1 and 1C4 conformations present but also skew conformations to some extent. Intramolecular hydrogen bonding was concluded to be important for the 3-C-methyl-substituted β-D-xylopyranosides in the non-polar solvent. Dynamic NMR (DNMR) spectroscopy was carried out for the 3-deoxygenated compound, which at 25 °C in methanol-d4 exists with equally populated states of the 4C1 and the 1C4 conformations, but at −100 °C only a few percent is present of the latter. Using 13C NMR detection for DNMR, resonance lines were shown to broaden at −40 °C and to sharpen again below −90 °C, without the emergence of a second set of NMR resonances, a typical behavior for an unequally populated equilibrium. The enthalpy and entropy activation barriers were calculated and resulted in ΔH‡ = 47.3 kJ mol−1 and ΔS‡ = 54 J mol−1 K−1.

 Please wait while we load your content...
Please wait while we load your content...