Lipase-catalyzed asymmetric synthesis of oxathiazinanones through dynamic covalent kinetic resolution†
Abstract
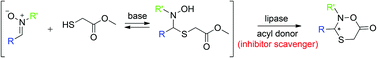
A domino addition–lactonization pathway has been applied to a dynamic covalent resolution protocol, leading to efficient oxathiazinanone formation as well as chiral discrimination. A new, double biocatalytic pathway has furthermore been proposed and evaluated where the initial product inhibition could be efficiently circumvented.

 Please wait while we load your content...
Please wait while we load your content...