An intramolecular cascade cyclization of 2-aryl indoles: efficient methods for the construction of 2,3-functionalized indolines and 3-indolinones†
Abstract
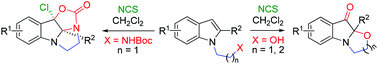
Efficient intramolecular N/O-nucleophilic cyclization of 2-aryl indoles has been developed to afford the corresponding 2-aza-3-oxaindolines and 3-indolinones in 80–95% yield. The methods provided convenient access to fused imidazo[1,2-c]oxazolidinone, oxazolidine, or tetrahydro-1,3-oxazine cores under mild conditions.

 Please wait while we load your content...
Please wait while we load your content...