Synthesis of the tumor associative α-aminooxy disaccharide of the TF antigen and its conjugation to a polysaccharide immune stimulant†
Abstract
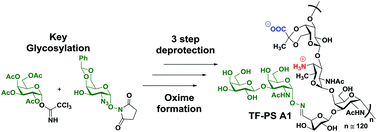
The α-aminooxy derivative of the Thomsen–Friedenriech tumor associated carbohydrate antigen has been synthesized in 11 steps utilizing a D-GalN3 acceptor carrying a pre-installed α-N-hydroxysuccinimidyl moiety. The natural α linkage was prepared in high selectivity employing a suitably protected D-GalN3-thioglycoside donor with N-hydroxysuccinimide. With access to α-TF-ONH2, the preparation of the TF-PS A1 vaccine candidate ensued smoothly through oxime bond formation.

 Please wait while we load your content...
Please wait while we load your content...