NHC-catalyzed oxidative cyclization reaction for the synthesis of 3-substituted phthalides†
Abstract
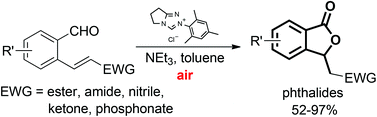
An efficient NHC-catalyzed domino oxidation/oxa-Michael addition reaction of 2-alkenylbenzaldehydes has been developed to afford 3-substituted phthalides bearing a C3-stereogenic center with a broad substrate scope and wide functional group tolerance. The preliminary results of the asymmetric process have been provided as well.

 Please wait while we load your content...
Please wait while we load your content...