Synthesis of β-carboline–benzimidazole conjugates using lanthanum nitrate as a catalyst and their biological evaluation†
Abstract
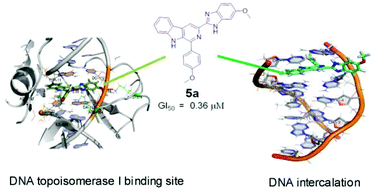
A series of β-carboline–benzimidazole conjugates bearing a substituted benzimidazole and an aryl ring at C3 and C1 respectively were designed and synthesized. The key step of their preparation was determined to involve condensation of substituted o-phenylenediamines with 1-(substituted phenyl)-9H-pyrido[3,4-b]indole-3-carbaldehyde using La(NO3)3·6H2O as a catalyst and their cytotoxic potential was evaluated. Conjugates 5a, 5d, 5h and 5r showed enhanced cytotoxic activity (GI50 values range from 0.3 to 7.1 μM in most of the human cancer cell lines) in comparison to some of the previously reported β-carboline derivatives. To substantiate the cytotoxic activity and to understand the nature of interaction of these conjugates with DNA, spectroscopy, DNA photocleavage and DNA topoisomerase I inhibition (topo-I) studies were performed. These conjugates (5a, 5d and 5r) effectively cleave pBR322 plasmid DNA in the presence of UV light. In addition, the effect of these conjugates on DNA Topo I inhibition was studied. The mode of binding of these new conjugates with DNA was also examined by using both biophysical as well as molecular docking studies, which supported their multiple modes of interaction with DNA. Moreover, an in silico study of these β-carboline–benzimidazole conjugates reveals that they possess drug-like properties.

 Please wait while we load your content...
Please wait while we load your content...