Probing the substrate specificity of Trypanosoma brucei GlcNAc-PI de-N-acetylase with synthetic substrate analogues†
Abstract
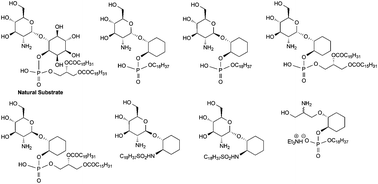
A series of synthetic analogues of 1-D-(2-amino-2-deoxy-α-D-glucopyranosyl)-myo-inositol 1-(1,2-di-O-hexadecanoyl-sn-glycerol 3-phosphate), consisting of 7 variants of either the D-myo-inositol, D-GlcpN or the phospholipid components, were prepared and tested as substrates and inhibitors of GlcNAc-PI de-N-acetylase, a genetically validated drug target enzyme responsible for the second step in the glycosylphosphatidylinositol (GPI) biosynthetic pathway of Trypanosoma brucei. The D-myo-inositol in the physiological substrate was successfully replaced by cyclohexanediol and is still a substrate for T. brucei GlcNAc-PI de-N-acetylase. However, this compound became sensitive to the stereochemistry of the glycoside linkage (the β-anomer was neither substrate or inhibitor) and the structure of the lipid moiety (the hexadecyl derivatives were inhibitors). Chemistry was successfully developed to replace the phosphate with a sulphonamide, but the compound was neither a substrate or an inhibitor, confirming the importance of the phosphate for molecular recognition. We also replaced the glucosamine by an acyclic analogue, but this also was inactive, both as a substrate and inhibitor. These findings add significantly to our understanding of substrate and inhibitor binding to the GlcNAc-PI de-N-acetylase enzyme and will have a bearing on the design of future inhibitors.

 Please wait while we load your content...
Please wait while we load your content...