Characterization of phenomena occurring at the interface of chiral conducting surfaces
Abstract
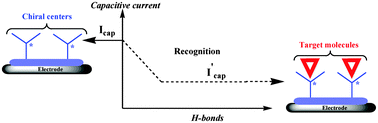
The ability of the chiral electrode based on L-leucine functionalized terthiophene (poly(1)-Pt) to recognize and detect biomolecules has been studied as a function of hydrogen bonding between the chiral surface and a free L-leucine methyl ester. We characterized electrochemically the formation of hydrogen bonds by cyclic voltammetry (CV). The results show that the capacitive current of the chiral electrode poly(1)-Pt decreased by 30% due to hydrogen bonding between the chiral electrode and the free added L-leucine methyl ester. The origin of the hydrogen bonds on poly(1)-Pt has been confirmed by attenuated total reflection Fourier transform infrared (ATR-FTIR) using trifluoroacetic acid (CF3COOH) as free H-bonding species. The ATR-FTIR spectrum exhibits functionalities of free CF3COOH that form hydrogen bonds with the chiral conducting surface of poly(1)-Pt. Due to the insolubility of poly(1), NMR studies were performed on the parent monomers. The chemical shift of the amide proton in the 1H-NMR of the L-leucine functionalized terthiophene (1) shifted after addition of L-leucine methyl ester. Similar trends were observed for the carboxylic carbonyl of L-leucine methyl ester-terthiophene 2 in the 13C-NMR. In addition to the change in the 13C chemical shift, there is a considerable change in the spin–lattice relaxation time of the carbonyl carbon in 2 due to the formation of hydrogen bonds between the –COOH of 2 and the imidazole.

 Please wait while we load your content...
Please wait while we load your content...