Synthesis of carboxylate functionalized A3B and A2B2 thiaporphyrins and their application in dye-sensitized solar cells†
Abstract
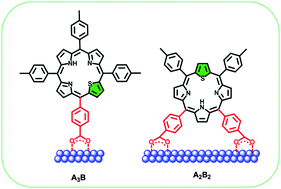
A series of novel A3B and A2B2 thiaporphyrins consisting of mono or dual anchoring groups with different linkers has been synthesized and effectively applied in dye-sensitized solar cells (DSSCs). The presence of an ethynylphenyl linker has a pronounced effect on their optical, electrochemical and photovoltaic properties. The ethynylphenyl linker bathochromically shifted the absorption spectra. Density functional theory (DFT) studies revealed that attachment of an ethynylphenyl linker through one of the meso positions to the porphyrin core results in planar macrocycles which is essential for the active electron coupling of the porphyrin core with the anchoring group in DSSCs. Although the dual anchoring groups can bind strongly to the TiO2 surface, the presence of the ethynylphenyl linker and especially the electron withdrawing cyano group on the anchoring group proved to be the pivotal factors to achieve higher efficiency. Among these dyes, N3S-ECN, having an ethynylphenyl linker and a cyano acrylic acid as the anchor, achieved the highest efficiency of 1.69%, with Jsc = 5.12 mA cm−2, Voc = 0.49 V and ff = 67%. To the best of our knowledge this is the highest efficiency obtained for thiaporphyrins.

 Please wait while we load your content...
Please wait while we load your content...